Difference Between Heat and Temperature

The concept of temperature and heat are studied together in science, which is somewhat related but not alike. The terms are very common, due to their extensive usage in our day to day life. There exists a fine line which demarcates heat from temperature, in the sense that heat is thought of, as a form of energy, but the temperature is a measure of energy.
The fundamental difference between heat and temperature is slight but significant, heat is the overall energy of the molecular motion, whereas temperature is the average energy of the molecular motion. So, let’s take a look at the article given below, in which we have simplified the two for you.
Definition of Heat
The heat of an object is the aggregate energy of all molecular movement inside the object. A form of energy that is transmitted from one object or source to another due to the differences in their temperature. It moves from a hotter object to the cooler one. Its measurement can be done in energy units, i.e. calorie or joules. The transfer of heat can take place in three ways, which are –
- Conduction: Heat transfer between molecules which are in direct contact with each other, without the movement of particles.
- Convection: The transfer of heat that takes place due to the movement of particles from one place to another is convection.
- Radiation: When the heat is transferred through a medium or vacuum, wherein space in between, is not heated up.
Definition of Temperature
Temperature is defined as the average kinetic energy of all molecules together, i.e. average energy of all the particles in an object. As an average measurement, the temperature of a substance does not rely on its size (number of particles) and type. It identifies how hot or cold an object is, in degrees. It also measures the speed of atoms and molecules of the substance.
Temperature can be measured in various scales, which are – Kelvin, Celsius and Fahrenheit. The thermometer is used to gauge the temperature of the object.
Important Differences Between Temperature and Heat.
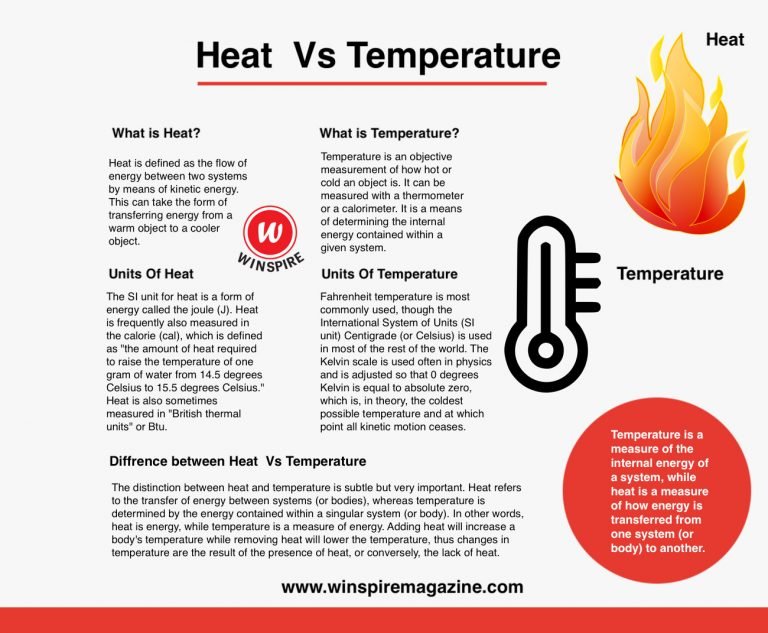
The differences between heat and temperature can be drawn clearly on the following grounds:
- Heat is nothing but the amount of energy in a body. As against this, the temperature is something that measures the intensity of heat.
- Heat measures both kinetic and potential energy contained by molecules in an object. On the other hand, temperature measures the average kinetic energy of molecules in a substance.
- The main feature of heat is that it travels from a hotter region to a colder region, whereas temperature rises when heated and falls when cooled.
- Heat possesses the ability to work, but the temperature is used exclusively to gauge the extent of heat.
- The standard unit of measurement of heat is Joules, while that of temperature is Kelvin, but it can also be measured in Celsius and Fahrenheit.
- The calorimeter is a device, which is used to measure the heat. On the other hand, the temperature can be measured by a thermometer.
- Heat is represented by ‘Q’ whereas ‘T’ is used to represent temperature.
Conclusion
Both heat and temperature are the concepts of thermodynamics; that works together to let the energy flow from the hotter body to the cooler body. While heat depends on the number of particles in an object, the temperature does not depend on several particles in an object because it is an average measurement.
It’s Questions Time.
- What is heat?
- What is SI unit of temperature?
- What is the relation between heat and temperature?
- What is your understanding about Kelvin?
- What do you convert Fahrenheit to Celsius?
We’ve created this content for informational purposes only, and it reflects the views of its respective authors/entities (freelancers/interns) and not those of Winspire Magazine. Winspire Magazine does not endorse or vouch for the accuracy of the information provided in this content. It is the reader’s responsibility to verify and ensure the information is correct and up-to-date. Winspire Magazine disclaims any liability or responsibility for any damages or losses from using this content. Therefore, readers should take all necessary steps to verify the accuracy and reliability of any information presented in this content.


